SUMMARY
This is AI generated summarization, which may have errors. For context, always refer to the full article.
MANILA, Philippines – The Food and Drug Administration (FDA) on Monday, March 30, approved 5 antibody test kits for COVID-19, the disease caused by the novel coronavirus.
In a press briefing, FDA Director-General Eric Domingo said the rapid test kits are also being used in coronavirus-hit countries with advanced technology such as Singapore and China.
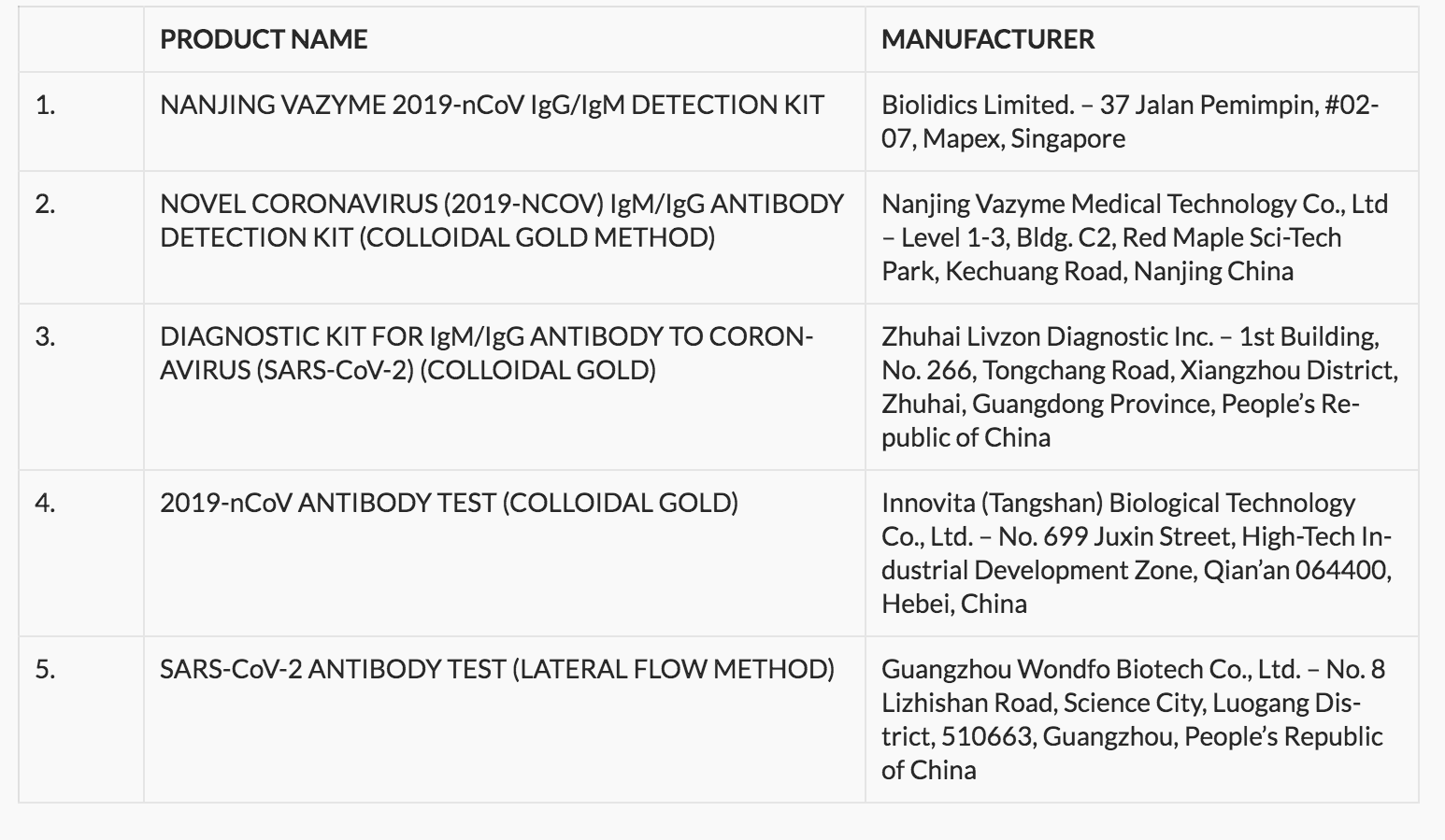
Below is the list of the rapid test kits approved by the FDA.
What’s the difference? The traditional PCR-based test kits use actual swabs from patients and determine the actual presence of the coronavirus, while rapid test kits require the patient’s blood sample and can only detect antibodies.
Domingo said rapid test kits will yield faster results compared to PCR-based test kits.
However, he noted that swabs would still be taken from patients for confirmatory testing using the standards of a PCR-based test.
“We have to be very cautious in using these rapid test kits because they measure antibodies and not the viral load itself,” Domingo said.
He further explained that the body takes time to develop antibodies and this “might give a negative result for patients who have been infected but bodies have not yet developed antibodies.”
Domingo called on the public to only use rapid test kits under the supervision of a medical professional. (READ: Coronavirus mass testing not needed for now – DOH)
“Nanawagan po kami na ang paggamit ng rapid testing ay dapat may pangangasiwa ng doctor or medical trained professional. Kailangan pong magawa ang test ng maayos at maging wasto ang interpretasyon ng resulta,” he added.
(We call on the public to use the rapid testing kits under the supervision of a doctor or a medical professional. The test should be done properly and the results should be interpreted accurately.)
While the health department rejected an earlier recommendation to use rapid test kits, Domingo said these kits can still be used in some cases, like in a community with a high number of suspected COVID-19 cases.
“Halimbawa, sa mga lugar na matagal nang umiikot ang virus at pinaghihinalaan na madaming tao na ang na impeksiyon o sa mga pasyenteng may mga malubhang sintomas ngunit walang paraan na mabilisang magpa-test sa mga DOH laboratory,” Domingo explained.
(For instance, in areas where the virus has been spreading for some time now and it is believed that many have already been infected, or for patients with severe symptoms but are unable to get immediate tests done at DOH laboratories.)
Domingo also announced that the FDA has approved the SARS-CoV-2 kit by Gene Xpert from Abbott Laboratories which can detect the virus within 5 minutes. This test kit is PCR-based.
The FDA has so far approved 17 PCR-based test kits for commercial use.
As of Sunday, March 29, the Philippines has 1,418 coronavirus cases, with 71 deaths and 42 recoveries. – Rappler.com
Add a comment
How does this make you feel?
There are no comments yet. Add your comment to start the conversation.