SUMMARY
This is AI generated summarization, which may have errors. For context, always refer to the full article.
The Department of Health (DOH) said 3 coronavirus vaccine manufacturers have passed the country’s ethics review board (ERB) – a requirement needed to conduct clinical trials in the Philippines.
In a virtual press briefing on Monday, December 7, Health Undersecretary Maria Rosario Vergeire said Europe-based vaccine makers Janssen of Johnson & Johnson and AstraZeneca, as well as China’s Clover Biopharmaceuticals got the approval of the ERB.
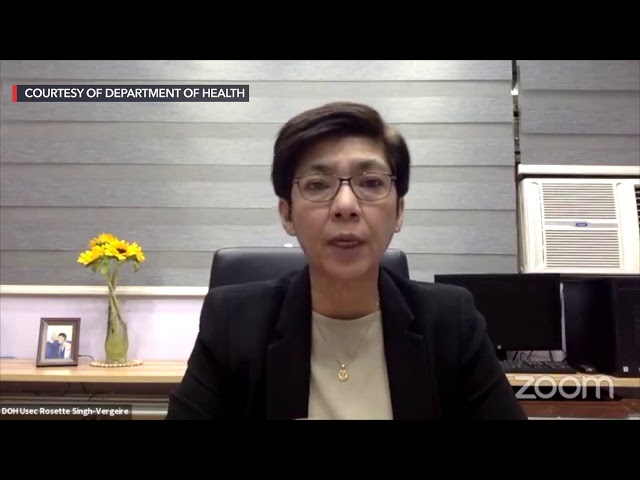
She reiterated Clover and another Chinese manufacturer, Sinovac, were already approved by the country’s vaccine expert panel (VEP), adding that “they are just undergoing the other processes before they can proceed with the FDA (Food and Drug Administration) authorization.”
Meanwhile, Jansen and AstraZeneca are still waiting for VEP’s approval.
Vaccine companies need to seek approval from the ERB and VEP before they could get approval from the FDA, which is tasked to regulate clinical trials in the country.
“As we always say, our pathway is parallel study of the vaccine expert panel and ethics board. If a company will not pass either of the two, it will not enter the FDA process. It needs to be approved by the VEP and ethics review board,” Vergeire added in a mix of English and Filipino.
However, she said that the FDA has been gathering information on these vaccines before to hasten their documentary evaluation.
Clinical trials are a type of research process that evaluates potential medical interventions that affect one’s health. During trials, medicines, treatments, procedures, and devices may be tested to study or verify their clinical or pharmacological effects before they are approved for public use.
Vergeire also said that the Philippine government has sent data confidentiality agreements with Moderna and Pfizer, two other frontrunners in vaccine development. However, Vergeire did not disclose the details of those agreements.
Philippine President Rodrigo Duterte on December 4 called for universal access to COVID-19 vaccines before the United Nations General Assembly, saying it would be a “gross injustice” if developing nations were left behind in the road to post-pandemic recovery.
The country has so far secured a supply of at least 2.6 million doses of the AstraZeneca vaccine, after the government and private firms signed a deal last November 27. – Rappler.com
Add a comment
How does this make you feel?
There are no comments yet. Add your comment to start the conversation.