SUMMARY
This is AI generated summarization, which may have errors. For context, always refer to the full article.

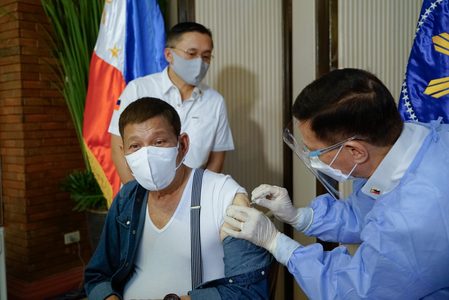
After President Rodrigo Duterte ordered the return of 1,000 donated Sinopharm vaccine doses, Malacañang clarified that one of those doses would remain in the country as the Philippine leader’s second dose.
“Hindi ibabalik ang second dose ni Presidente para matapos niya ‘yung second dose (The second dose of the President won’t be returned so he can complete the second dose),” Presidential Spokesman Harry Roque said on Thursday, May 6, during a virtual press briefing.
Duterte had ordered the return of the donation after critics asked why he had opted to receive a COVID-19 jab not yet covered by emergency use authorization from the Philippines’ Food and Drug Administration.
The dose Duterte received was covered only by a compassionate special permit that allows only the Presidential Security Group (PSG) hospital to administer Sinopharm vaccine shots.
Roque said, however, that the President is still open to Filipinos using the Sinopharm vaccine in the future, but only if it is covered by an EUA.
The Duterte spokesman was also asked why Duterte chose the Sinopharm vaccine when a World Health Organization Strategic Advisory Group of Experts had only “low confidence” in the quality of evidence that the jab could prevent COVID-19 among adults aged 60 and above. Duterte is 76.
Roque said, “That’s for his doctors to answer, that’s why the President said to just give it back and let the EUA process continue.”
Sinopharm has yet to even apply for a Philippine EUA. The FDA has said two agencies claiming to represent the state-owned firm are yet to complete the documents required for a full review. – Rappler.com
Add a comment
How does this make you feel?


There are no comments yet. Add your comment to start the conversation.