SUMMARY
This is AI generated summarization, which may have errors. For context, always refer to the full article.
![[OPINION] Getting up close and personal with the atom, and its nucleus that powers NPPs](https://www.rappler.com/tachyon/2021/12/Screen-Shot-2021-12-16-at-4.31.11-PM.png)
The following is the 20th in a series of excerpts from Kelvin Rodolfo’s ongoing book project “Tilting at the Monster of Morong: Forays Against the Bataan Nuclear Power Plant and Global Nuclear Energy.“
Our next four forays are meant to provide some necessary background for readers who know little science, so they can better understand how nuclear power plants work and the dangers they pose. If you find atoms, chemistry and radiation forbiddingly complicated, this is for you. If you are fairly comfortable with chemistry and physics, you may wish to skip these forays. But this is your chance to correct me and measure how much better informed you are.
‘Atom?’
Since the 5th Century BC, people have made atomic models with the knowledge available to them. Greek philosophers first arrived at the idea. A beach is many sand grains; a sand grain can be crushed. A cup of water can be divided into drops, a drop into droplets. Can matter be subdivided indefinitely? No, they decided, or eventually “something” would become “nothing.” Therefore, matter must be made up of very small atoma – literally, “cannot-be-cuts” in Greek.
Science changed that understanding in World War II when it used very complicated technology to release huge amounts of energy by splitting atoms in nuclear bombs.
Atoms are entirely made of energy! Only energy existed when the Universe began. As it cooled, some energy began concentrating into particles that make up matter.
Let’s translate Einstein’s famous E= mc2 into words: “Huge amounts of energy equal small amounts of matter.” “Huge” is c2, the velocity of light c – an already enormous number – multiplied against itself! Theoretically, if the roughly five grams of your little finger could be completely converted into energy, it could power 250 city-dwellers for a whole year.
But that is only theoretical. The best a nuclear power plant like BNPP can do is to very inefficiently convert very small amounts of uranium mass into energy.
Our own atomic model
No atomic model is “real.” Each, including the most modern, is only a tool for studying limited aspects of sub-microscopic reality. So, let’s make our model as simple as possible and only as complex as we need.
Let’s approach the atom from its outside, because its outermost parts determine which element every atom belongs to: how it combines with others to make substances like air, water, rock, our own living tissue. But we won’t linger there; our main goal is the nucleus in the deep atomic interior.
To make our model atom, we will need only three kinds of smaller, “sub-atomic” particles that were already known in the 1930’s. The familiar atomic logos all show a few outside electrons looping around a tiny nucleus. Here’s my personal favorite, because its electrons make the atom seem so exuberantly alive:
Such logos are much too crude to show that the central nucleus is actually made of two kinds of smaller “sub-atomic” particles: protons and neutrons, which we will look at after discussing electrons. Those three sub-atomic particles are all we need for our purposes, even though physicists have dissected the atom far beyond that.
Electrons
“Electricity” and “electron” come to us from elektron, “amber” in Greek. The ancient Greeks knew that if you rub a piece of amber with wool it strangely attracts things like bits of paper. Have you ever noticed while combing your hair that your comb begins to behave like a magnet, attracting strands of hair?
Early in the 18th Century, scientists determined that there are only two kinds of electrical charge. Objects with the same charge repel each other; those with opposite charge are attracted to each other. Two amber rods rubbed with wool repel each other because they both have what scientists decided to call negative charge. But one of these rods will attract a glass rod that has become positively charged by being rubbed with silk.
Rubbing pieces of amber with wool or glass with silk to give them electric charge doesn’t make them lose or gain weight, and so the tiny bits of charge that they exchange must be almost weightless. So just think of the electron as the smallest possible bit of electric energy, with a negative value of “one.” Its mass is so small it weighs practically nothing.
The electrons of an atom are no different than those that stream out of wall outlets as electricity; it’s just that they are attracted and held to the atom by positive charges in the nucleus.
The mass of every atom, like the meat in a nut, is in its kernel, “nucleus” in Latin. The nucleus is surrounded by one or more “shells” of electrons. How many electrons there are in the outermost or “valence” shells of the different elements governs how they join each other to make different stuffs. For example, how two hydrogens make a threesome with an oxygen to become an H2O water molecule, or how two oxygens join a carbon to make one of CO2:

Chemical changes consist of breaking apart the outer-shell bonds between atoms, and making them bond with other atoms to form new stuff, as when wood burns with oxygen into ash and smoke. Our very existence is all outer-shell chemistry: how plants photosynthesize our food with sunlight, how we release and use that stored energy by digesting it, how your blood uses iron to capture oxygen and release it wherever your body needs it.
But that familiar sort of valence chemistry is not what nuclear power is about, which is why we won’t get into outer-shell valence chemistry more closely here.
Welcome to the strange world of the inner atom and the nuclear chemistry that would power BNPP, by seeing how tiny a nucleus is, compared to the whole atom.
The Size of the Nucleus
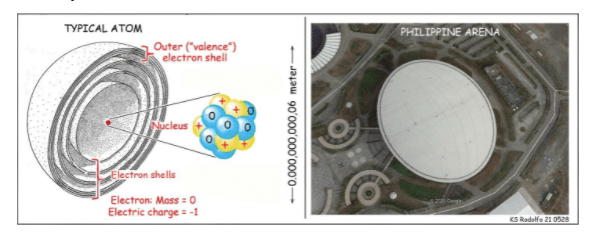
Truth is, we cannot draw a faithful picture of an atom. The one on the left is no exception. An atom has no color; it’s too small, relative to light waves. Its outer “shells” are actually clouds of electrons in furious motion. And diameters of typical atoms are measured in trillionths of a meter; their nuclei (plural of nucleus) are about 10,000 times smaller!
A large true-scale model of an atom and its nucleus helps to illustrate that huge size difference. For our model atom’s outer surface, let’s use the dome roof of the Iglesia Ni Cristo’s Philippine Arena in Bulacan that seats 55,000 people.
The dome is 170 meters long; pretend it’s spherical. The nucleus is far too small to draw. But with your mind’s eye, look down through the center of the dome, down to the center of the arena floor.
There: See? Our model nucleus, only as big as a ping-pong ball.
Isn’t it strange that the atoms that make up everything – air, water, metals, rocks, your own body – consist mostly of empty space between the electron shells of atoms and their nuclei?
Before we leave electron-shell chemistry: The number of electrons that each atom holds, and governs how it behaves chemically, is not accidental. It matches the number of positively-charged protons in the nucleus, the yellow balls in the drawing. That is its atomic number. Examples: Carbon 6, Oxygen 8, Thorium 90, Uranium 92, Plutonium 94.
Our next foray discusses protons, and neutrons, the blue balls marked “O.” – Rappler.com
Born in Manila and educated at UP Diliman and the University of Southern California, Dr. Kelvin Rodolfo taught geology and environmental science at the University of Illinois at Chicago since 1966. He specialized in Philippine natural hazards since the 1980s.
Keep posted on Rappler for the next installment of Rodolfo’s series.
Previous pieces from Tilting at the Monster of Morong:
- [OPINION] Tilting at the Monster of Morong
- [OPINION] Mount Natib and her sisters
- [OPINION] Sear, kill, obliterate: On pyroclastic flows and surges
- [OPINION] Beneath the waters of Subic Bay an old pyroclastic-flow deposit, and many faults
- [OPINION] Propaganda about faulting, earthquakes, and the Bataan Nuclear Power Plant
- [OPINION] Discovering the Lubao Fault
- [OPINION] The Lubao Fault at BNPP, and the volcanic threats there
- [OPINION] How Natib volcano and her 2 sisters came to be
- [OPINION] More BNPP threats: A Manila Trench megathrust earthquake and its tsunamis
- [OPINION] Shoddy, shoddy, shoddy: How they built the Bataan Nuclear Power Plant
- [OPINION] Where, oh where, would BNPP’s fuel come from?
- [OPINION] ‘Megatons to Megawatts’: Prices and true costs of nuclear energy
- [OPINION] Uranium enrichment for energy leads to enrichment for weapons
- [OPINION] Introducing the nuclear fuel cycle
- [OPINION] On uranium mining and milling
- [OPINION] Enriching and fabricating BNPP’s uranium fuel
- [OPINION] Decommissioning BNPP, and storing the nuclear dragon’s radioactive manure
- [OPINION] So how much greenhouse gas does nuclear power really generate?
Add a comment
How does this make you feel?
There are no comments yet. Add your comment to start the conversation.