SUMMARY
This is AI generated summarization, which may have errors. For context, always refer to the full article.
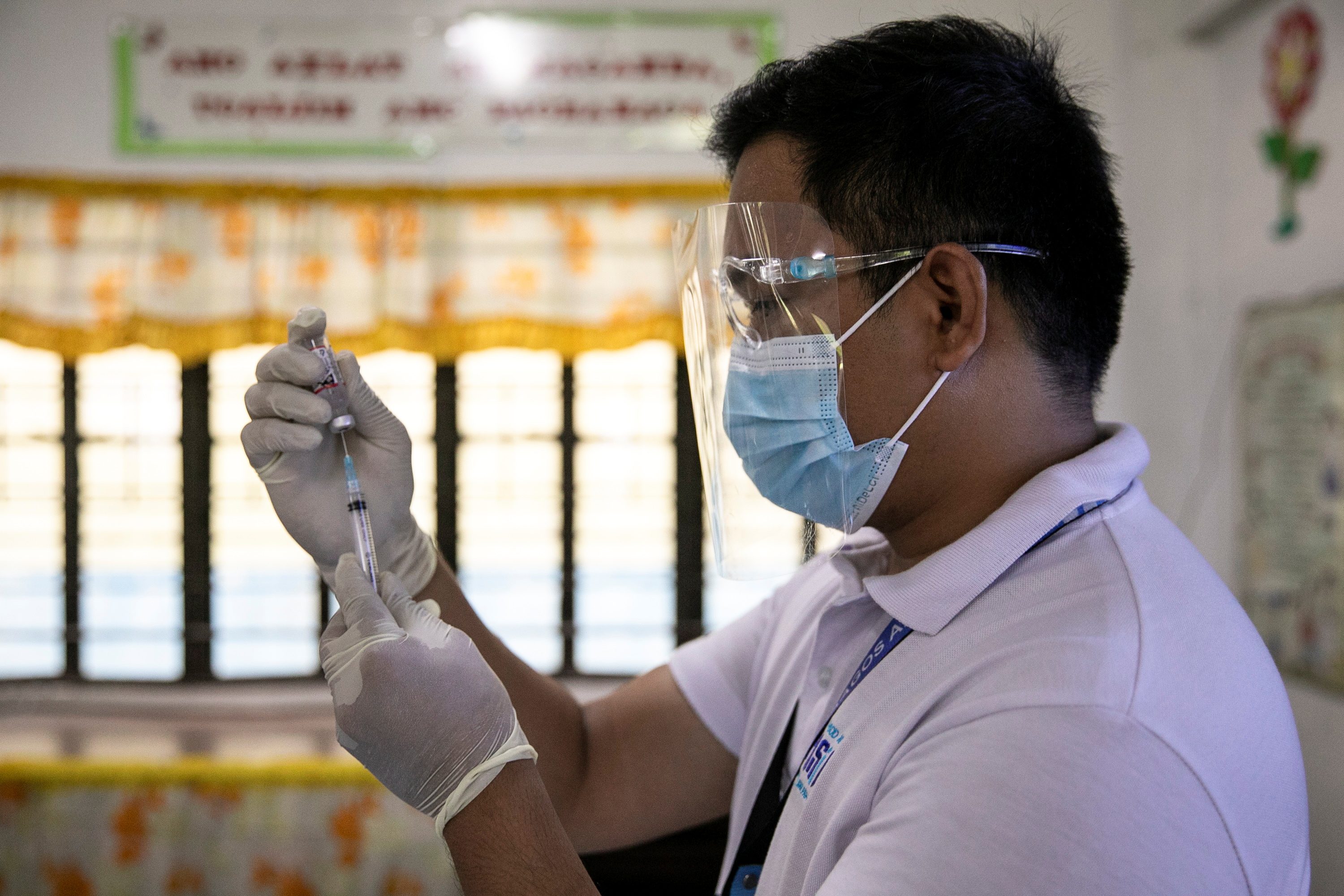
Philippine pandemic officials announced the delivery of the country’s first batch of coronavirus vaccines could be expected by mid-February. But with that timeline looking less likely to be met, the Duterte government’s task force officials announced there would be a slight delay in the arrival of vaccines due to, of all things, paperwork.
Vaccine czar Carlito Galvez Jr and National Task Force COVID-19 Deputy Chief Implementer Vince Dizon pointed to documentary requirements involving an indemnification fund as the culprit behind the delayed schedule.
Why do we need an indemnification fund?
An indemnification fund, Galvez earlier told lawmakers during congressional hearings, was necessary for the Philippines to gain access to vaccine doses from pharmaceutical companies as well as the COVAX global facility.
He requested lawmakers to consider bills that would provide for such in mid-January, adding it could give the Philippines an opportunity to gain access to larger supplies of vaccines and increase public confidence in the government’s rollout.
The reason why indemnification funds or programs are required of countries is because vaccines being used worldwide are still under emergency use authorization (EUA), with only limited short-term data available on the full effects that could arise from receiving a shot.
Under indemnification programs, governments agree that distributing entities like COVAX, along with vaccine manufacturers, will not be held liable for unexpected adverse events. This is a risk governments absorb during the pandemic when granting vaccines emergency use approval.
“You’ll have an indemnification program because there is no full data yet. So if an unexpected adverse event happens, any company cannot be sued because you enter an agreement with the company where you are guaranteeing them that you will take responsibility for any adverse event because you’re aware the data is not complete yet – but you’re allowing its use because there is a national emergency,” said Dr Aileen Espina, a public health and family medicine specialist and member of the Healthcare Professionals Alliance Against COVID-19 (HPAAC).
“That’s why we’re particular that for any vaccine that will be used, the benefits outweigh the risks,” she added.
But vaccines aren’t here yet. What else should the government do?
On Monday night, February 15, Galvez said that task force for vaccine procurement officials have done their part in submitting requirements needed to facilitate Filipinos’ access to vaccines. This is on top of efforts by the Department of Health.
Specifically, government lawyers have finished fine-tuning details of an indemnification clause with COVAX and are now waiting on the facility, the World Health Organization (WHO), and Pfizer to finalize it. (Among the vaccines expected to arrive in the Philippines in the first quarter of 2021 are those developed by Pfizer, BioNTech, and AstraZeneca.)
On Wednesday, February 17, Galvez said that the Philippine government has already signed onto the agreements.
The vaccine czar said it was now waiting on Congress and the WHO to ensure the availability of an indemnification fund and concur with the Philippines’ proposed indemnification program, respectively.
“Nandoon po sa Congress ang ball at saka po nandoon po sa WHO (The ball is with Congress and the WHO),” Galvez said.
While requirements for vaccines coming from COVAX are steps away from being finalized, lawmakers in Congress will still need to pass measures creating an indemnification program to facilitate access to vaccines purchased through agreements between the Philippines and vaccine manufacturers.
Why are we talking about indemnification only now if it’s important?
The government was informed of the indemnification requirement only during the latter phase of vaccine negotiations.
Senator Sonny Angara said on Wednesday, February 17, that this issue was not even raised during the 2021 budget deliberations, “otherwise, [Congress] would have put a fund on that.”
Galvez first informed the Senate about the need for legislation on the indemnification program during committee of the whole hearings in January. It was only on February 11 that the urgency of this measure was tackled in depth during a public hearing.
Nandoon po sa Congress ang ball at saka po nandoon po sa WHO.
Secretary Carlito Galvez Jr
Why do we need a law? Won’t this further delay vaccine arrival?
A law is needed to be able to tap into the Contingent Fund, as this expense was not covered under the GAA, Budget Secretary Wendel Avisado told Rappler.
National Task Force COVID-19 deputy implementer Vince Dizon said a law is also being required by vaccine manufacturers, aside from the COVAX facility, to hold them free from any liabilities for severe adverse effects arising from immunization.
COVAX officials earlier listed this as part of requirements for all countries participating in the global scheme to ensure equitable access to vaccines, saying vaccine manufacturers were reluctant to guarantee supplies if they were not indemnified against possible liability.
“Given the unprecedented speed and scope of deployment of recently licensed vaccines, manufacturers have highlighted the risks of unforeseen claims due to injury associated with the vaccine. Manufacturers are reluctant to deliver COVID-19 vaccines if this risk is not addressed,” COVAX said.
For countries like the Philippines that will receive donated vaccines from the facility, COVAX said a model indemnification agreement will already be incorporated in agreements with manufacturers to decrease time spent for negotiations.
Where will the government source indemnity payments?
Since there was no allocation in the P4.5-trillion 2021 budget, the Philippine government and Congress have to look where they could source this.
During a February 11 Senate hearing, two options were considered:
- For the indemnity payments to be included under PhilHealth’s coverage using its funds
- Tapping into the P13-billion Contingent Fund in the national budget, which would require a law
During that hearing, Health Secretary Francisco Duque III said that PhilHealth did not include this in its corporate budget this year, but that the state health insurer will “study” the possibility of tapping into its P116-billion reserve funds.
The result of the February 11 hearing is Senate Bill (SB) 2057, which is currently being tackled at the upper chamber’s plenary. The bill is proposing the creation of a P500-million indemnification fund to be sourced from the Contingent Fund. This will cover compensation in case of death and medical treatment for vaccine recipients experiencing severe adverse effects.
In the House of Representatives, lawmakers have also filed a counterpart measure through House Bill No. (HB) 8648, which seeks to exempt local government units from bidding requirements under the procurement law, grant tax exemptions for vaccines, and create an indemnification fund for the country’s COVID-19 vaccine program.
So far, HB 8648 does not include an immunity clause, nor an amount that will be allocated for the indemnification fund.
Who will implement the indemnification program?
According to Senate Majority Leader Juan Miguel Zubiri, while funds will be administered by PhilHealth, the DOH will be in charge of implementing the indemnification program where adverse events linked to a vaccine may be determined.
This means that if someone were to experience a serious adverse reaction, the DOH, through its post-monitoring of vaccinations, will determine if an adverse effect is indeed linked to the vaccine. If so, PhilHealth would cover the cost of care, with funds sourced from the indemnity fund.
What’s the status of pending bills in Congress?
Both Senate and House bills were already sponsored at the plenary. At the upper chamber, interpellations will resume on Monday, February 22, before the Senate version of the indemnification bill is scheduled to be passed on 2nd reading.
At the House, amendments to HB 8648 were finalized on Wednesday. It is also up for 2nd reading.
At their current pace, the bills could be passed on final reading during the last week of February or during the first week of March due to the 3-day interval rule between the 2nd and 3rd reading. Since the current versions of the Senate and House bills are different, there’s also a chance of a bicameral conference committee (bicam).
If President Rodrigo Duterte certifies the vaccine bill as urgent – as requested by Galvez – Congress can do away with the 3-day interval rule.
If the President issues the certification in the last week of February, then there’s a chance that both chambers will also be able to pass the bills on final reading that same week. To further hasten the process, the Senate or the House may need to adopt the other chamber’s version, so that a bicam won’t be needed. The bill will then be up for signature by the President for it to become law.
Once a law is passed, will we get our vaccine supply immediately?
This will still depend on when the government will sign supply agreements with vaccine firms, as well as the capacity of vaccine manufacturers to deliver doses to all countries that have locked in orders.
As it stands, Galvez said only term sheets have been signed with vaccine manufacturers as of mid-February. Term sheets are the second to the last step in vaccine negotiations required to lock in logistics needed for production and delivery.
For the last step, a supply agreement will still need to be signed with these companies to facilitate actual purchase and payment, as well as set a definite date for delivery.
Galvez earlier said that current limited global supply has prevented vaccine manufacturers from entering binding supply agreements with the government. – with reports from Mara Cepeda/Rappler.com
Add a comment
How does this make you feel?



![[OPINION] The First Mode conundrum](https://www.rappler.com/tachyon/2024/03/tl-first-mode-conundrum-03232024.jpg?resize=257%2C257&crop=283px%2C0px%2C720px%2C720px)






There are no comments yet. Add your comment to start the conversation.