SUMMARY
This is AI generated summarization, which may have errors. For context, always refer to the full article.

For every stage of the Philippines’ coronavirus vaccine program, 5 groups of experts are tasked to guide government actions and assess the safety and efficacy of COVID-19 jabs.
The groups – some of which were formed only during the pandemic – are responsible for crucial steps, like creating a roster of vaccines for possible purchase, reviewing shots for emergency use, and how to best distribute the scarce supplies.
They are also entrusted with sifting through data on vaccines from different countries, study adverse events following immunization, and recommend how to adjust the use of shots to respond to urgent needs.
Among the recent moves of vaccine experts’ groups was to recommend the use of Sinovac’s vaccine on senior citizens and keep tabs of its possible side effects.
The Philippines’ 5 vaccine experts’ groups count among its members specialists in the fields of infectious diseases, vaccinology, public health, and epidemiology, among others. They also have among them ethicists, health economists, and sociologists. Together, they ensure the safety and efficacy of vaccines to be used in the Philippines.
Research and Development
VACCINE EXPERT PANEL
The vaccine expert panel (VEP), which is under the Department of Science and Technology (DOST), evaluates, selects, and creates a priority list of coronavirus vaccines the government could purchase.
The identities of the 9 VEP members are kept confidential to avoid “undue influence” in the evaluation and shortlisting of vaccines, Executive Director Jaime Montoya of the DOST Philippine Council Health Research and Development (PCHRD) earlier said.
The DOST and Department of Health (DOH) have only disclosed the identity of at least two members of the panel: VEP head Dr Nina Gloriani, who is the former dean of the University of the Philippine College of Public Health; and Dr Rontgene Solante, head of adult infectious diseases at the San Lazaro Hospital.
Based on a Philippine Council Health Research and Development order, the VEP is tasked with the following:
- Identify and evaluate candidate vaccines from local and international partners of the DOST.
- Identify local partners for the conduct of pre-clinical and clinical trials of vaccines in the country.
- Provide recommendations and action plans on the engagement of partners, pre-clinical and clinical trials’ requirements of the Food and Drug Administration (FDA), DOH, and World Health Organization (WHO).
- Evaluate project proposals on vaccine development seeking financial assistance from PCHRD or DOST.
- Assist in evaluating applications for emergency use authorization of vaccines in the Philippines.
Gloriani says that, in evaluating vaccines, the group uses the WHO’s criteria for which vaccines to prioritize. These are safety, efficacy, stability or storage conditions, implementation (how the vaccine can be distributed and administered), and availability or supply. The group also reviews scientific publications to back up its evaluations.
The VEP takes into account data available on candidate vaccines from pre-clinical studies, Phase 1, 2, and preliminary Phase 3 clinical trial results, and whether they have been granted emergency use authorizations in other countries.
Among other things, the VEP also considers the technology of vaccines, their ingredients, which may signal safety concerns, and the track record of companies developing vaccines, Gloriani says.
These are the criteria used by the Philippines’ National COVID-19 Vaccination Plan:
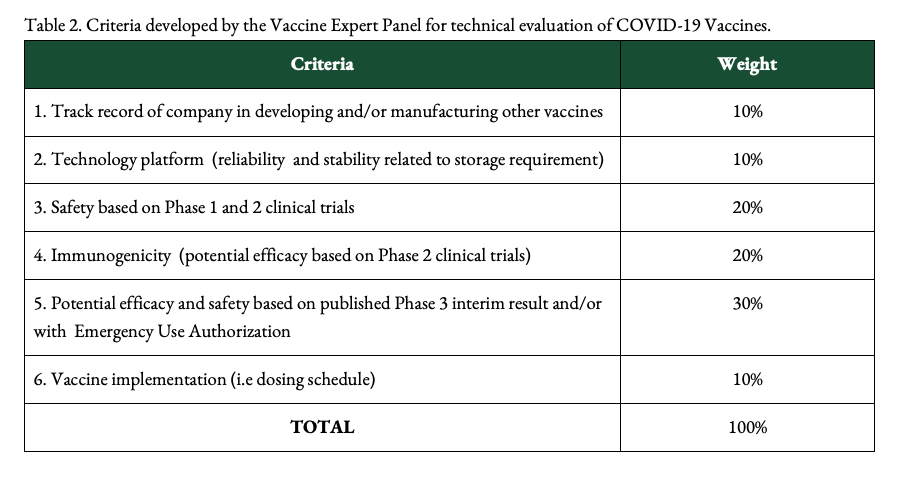
The VEP’s technical evaluation is the initial step. After this, vaccines eyed for use in the Philippine are reviewed by the Single Joint Research Ethics Board and the FDA for either late-stage trials or emergency use.
It is also used to guide the negotiations of Philippine vaccine czar Carlito Galvez Jr.
From a roster of over 230 COVID-19 vaccines being developed, the following were shortlisted by the VEP as of January 2021:

The evaluation and shortlist of vaccines, Gloriani says, will be updated as more scientific data and evidence become available.
Regulatory
The FDA’s expert panel is responsible for assessing the quality and efficacy of vaccines for emergency use.
The Health Technology Assessment Council (HTAC), meanwhile, is tasked with determining the cost efficiency and appropriateness of vaccines for use in a public health program, as well as any potential commercial or political conflicts of interest.
FDA EXPERT PANEL
The panel – whose membership is “strictly confidential” – is composed of at least 3 experts on drugs and vaccine development, immunology, infectious disease pharmacology, and public health toxicology.
When the FDA was given the authority to grant emergency use of COVID-19 shots and other medicines, among the requirements was for it to have a separate panel of experts to review data on the efficacy and safety of vaccines.
Once an application is filed with the FDA, documents are referred to the agency’s Center for Drug Regulation and Research (CDRR) and the FDA experts’ panel formed specifically for the review of vaccines seeking emergency use authorizations (EUAs).
The CDRR’s review of the technical aspects or quality of the vaccine and how it is manufactured, and the expert panel’s review of its safety and efficacy, are done simultaneously.
Based on evidence, the panel determines whether it is “reasonable to believe” that a drug or vaccine may be effective in preventing, diagnosing, and treating COVID-19. The group will also weigh the known and potential benefits of a vaccine or drug with its risks, if any.
Once reviews are completed, the expert panel crafts recommendations to the FDA director general, who decides whether an EUA will be issued.
HTAC
The HTAC, created by the Universal Health Care Law, started operating just as the COVID-19 pandemic took hold of the world in 2020. The council is a recommending body composed of an independent panel of experts. It assesses which health technologies should be funded or covered by taxpayers’ money under the DOH and Philippine Health Insurance Corporation (PhilHealth).
It reviews medical technology like tests, vaccines, medicines, and equipment “with the aim to improve overall health outcomes and ensure fairness, equity, and sustainability of coverage for all Filipino citizens.” Its assessments take into consideration “ethical, legal, social and health system implications.”
In the pandemic, the HTAC is most known for being the last step in approving the purchase of COVID-19 vaccines.
Once a vaccine is approved for emergency use by the FDA, the HTAC assesses its cost-effectiveness, potential conflicts of interests, and whether it can be bought by the DOH. When making an assessment, among the questions it asks are:
- Whether the vaccine can significantly reduce the magnitude and severity of COVID-19.
- If it is efficacious and safe.
- If it is affordable and feasible to use in a national immunization program.
- If it has the potential to reduce out-of-pocket expenses of Filipinos due to COVID-19.
- If it reduces or does not add to existing social inequities in the health system.
The DOH earlier asserted the HTAC’s recommendation would be mandatory under the Universal Health Care Law. However, the issue had been debated after Presidential Spokesperson Harry Roque said its recommendation was not required to secure COVID-19 vaccines.
In a televised address in early March, Health Secretary Francisco Duque III told President Rodrigo Duterte that the DOH’s legal division said the HTAC’s recommendation was necessary. “Absent this positive recommendation, the government cannot spend money on this technology or development of any benefit package by the DOH and PhilHealth,” Duque said.
Vaccination
National Immunization Technical Advisory Group (NITAG)
The NITAG serves as another recommendatory group to the DOH and Duterte government’s vaccine cluster, involving the actual implementation of COVID-19 vaccination.
It helps formulate policies, plans, and strategies in rolling out scarce shots.
The NITAG is the group that crafted the government’s list of priority groups to be vaccinated. The overall goal is to reduce deaths, decrease hospitalizations, protect vulnerable and high-risk groups, and, eventually, help tame the pandemic. The prioritization of vaccines was guided by the Strategic Advisory Group of Experts (SAGE) on Immunization, an advisory committee to the WHO.
The NITAG helps the DOH form guidelines for vaccination for local government units and health centers.
National Adverse Events Following Immunization Committee(NAEFIC)
The final expert group involved in the government’s COVID-19 vaccine program is the NAEFIC. It is another independent body responsible for assessing adverse events following immunizations.
Specifically, the NAEFIC studies whether there is evidence to determine a link between vaccines and serious adverse events.
Based on the final cause determined, the NAEFIC provides recommendations to the DOH Epidemiology Bureau, the FDA, the National Immunization Program, and centers for health development or regional offices.
The group also provides guidance to health officials on how to improve the delivery of immunization services, safety-related performance of vaccines, and management of patients experiencing serious side effects.
In line with the pandemic, the NAEFIC was restructured in 2020 to serve as an independent expert panel and to include among its members experts in the fields of pediatric infectious diseases, vaccinology, forensic pathology, immunology, allergology, and geriatrics, among others. – Rappler.com
Read Rappler’s series of explainers on the Duterte government’s vaccine program below:
- SCHEDULE: Philippines’ COVID-19 vaccine deliveries
- TIMELINE: The Philippines’ 2021 COVID-19 vaccine plan
- EXPLAINER: What to expect once COVID-19 vaccines arrive in the Philippines
- EXPLAINER: How COVID-19 vaccines will get from warehouses to you
- Securing vaccine deals: A checklist for local governments
- TRACKER: Which COVID-19 vaccines are being eyed by the Philippines?
- How FDA grants emergency approval for COVID-19 vaccines, meds
- PH to prioritize high-risk areas, sectors for COVID-19 vaccine rollout
- Gov’t releases new vaccine priority list, includes persons with comorbidities
- MAP: Which countries have started their COVID-19 vaccination program?
- LIST: Local governments’ plans, deals, and budget for COVID-19 vaccines
- Philippines targets purchase of 148 million doses of COVID-19 vaccine in 2021
- FAST FACTS: Prioritized groups, guidelines for COVID-19 vaccination
- Your guide to COVID-19 vaccination for seniors, persons with comorbidities
- LIST: 13 sectors eligible to get COVID-19 vaccine as economic frontliners
Add a comment
How does this make you feel?





There are no comments yet. Add your comment to start the conversation.