SUMMARY
This is AI generated summarization, which may have errors. For context, always refer to the full article.
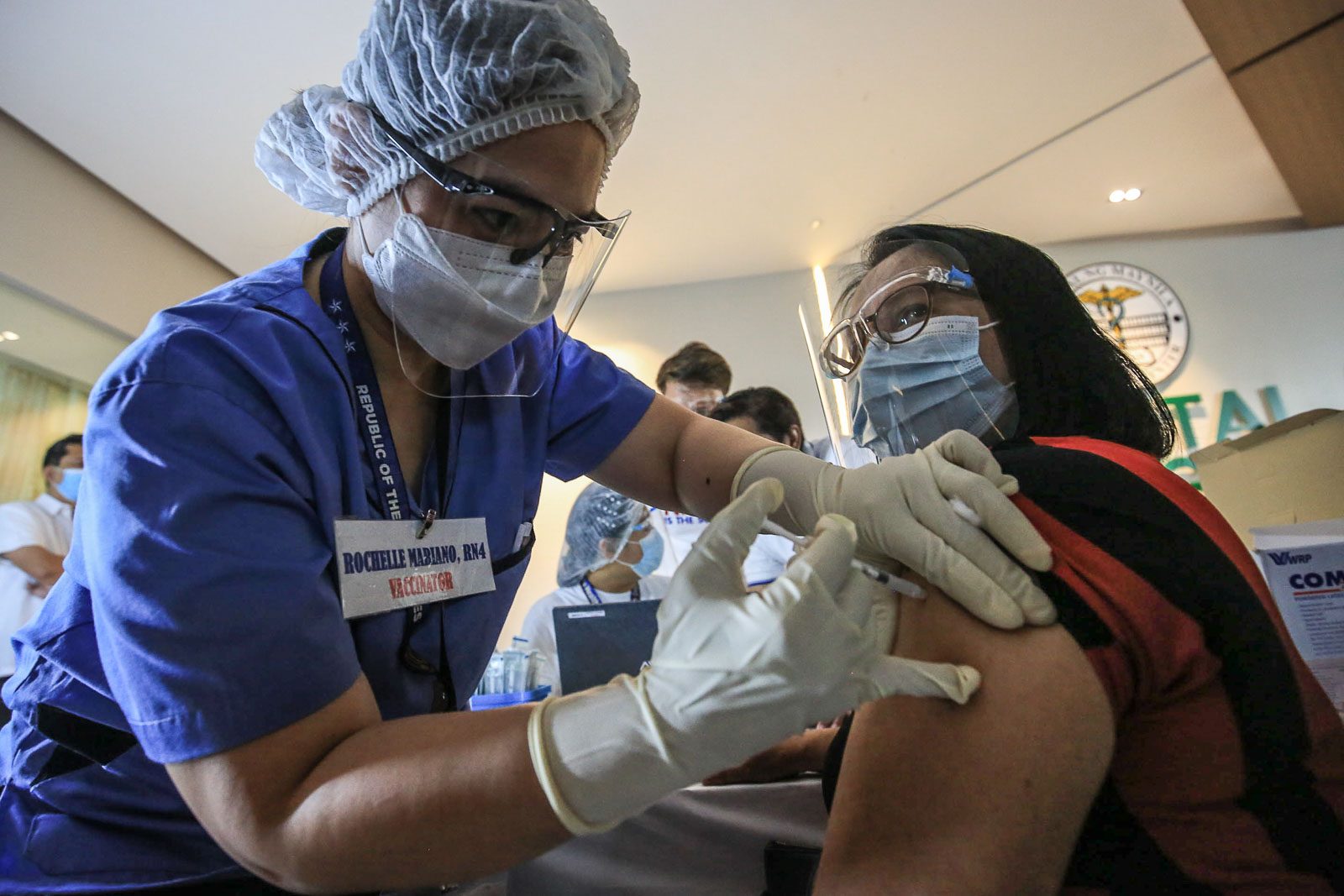
On March 1, the Philippines began its official COVID-19 vaccination campaign, which aims to inoculate at least 50 million Filipinos.
As of Thursday, March 11, the only COVID-19 vaccines that have been granted emergency approval by the Philippine Food and Drug Administration (FDA) are those produced by Pfizer-BioNTech, Sinovac, and Oxford-AstraZeneca.
The 2021 vaccine deployment plan was launched with the distribution of 600,000 doses of the Sinovac vaccine on February 28.
The immunization drive, however, has been marred by illegal vaccinations of people close to President Rodrigo Duterte. On February 23, a week before its launch, Duterte’s former special envoy to China, Ramon Tulfo Jr said that he and members of the Presidential Security Group (PSG), a “Cabinet member,” a senator, and several other government officials had already been vaccinated with Sinopharm jabs.
The vaccination campaign, expected to be bolstered by more doses of the vaccines arriving throughout 2021, prioritizes frontline workers in health facilities, senior citizens, and persons with comorbidities.
Below, we lay out the announced tiers of prioritization for COVID-19 vaccination and general guidelines to consider before getting the jab from any brand.
Who is prioritized for COVID-19 vaccination?
To ensure fair allocation of the vaccine, the list below was created by the National Immunization Technical Advisory Group (NITAG), a group of health experts advising the Inter-Agency Task Force on Emerging Infectious Diseases (IATF).
- A1: Frontline workers in health facilities both national and local, private and public, health professionals and non-professionals like students, nursing aides, janitors, barangay health workers, etc.
- A2: Senior citizens aged 60 years old and above
- A3: Persons with comorbidities not otherwise included in the preceding categories
- A4: Frontline personnel in essential sectors, including uniformed personnel and those in working sectors identified by the IATF as essential during ECQ
- A5: Indigent populations not otherwise included in the preceding categories
- B1: Teachers, social workers
- B2: Other government workers
- B3: Other essential workers
- B4: Socio-demographic groups at significantly higher risk other than senior citizens and indigent people
- B5: Overseas Filipino Workers
- B6: Other remaining workforce
- C: Rest of the Filipino population not otherwise included in the above groups
Prioritization was based on assessing risks of exposure and physical condition in compliance with the WHO’s recommendations of giving priority to medical frontliners and those considered “high-risk.” High-risk individuals are senior citizens, people with heart or lung disease, diabetes, or conditions affecting their immune system.
Generally, it is recommended to extensively consult a doctor regarding eligibility to receive the vaccine, especially if there are any prior health conditions and to consider guidelines of the specific brand of vaccine being used.
Guidelines for all COVID-19 vaccines
Now that you know where you lie in terms of prioritization for a COVID-19 vaccine, what else should you consider before getting the jab?
NITAG says that for the following groups, vaccination may be received once medical clearance is obtained:
- People with autoimmune disease, if in remission
- People living with HIV, if the patient’s current CD4 count is low and if the patient is on treatment
- People with cancer or malignancy, if the person is undergoing or has plans for chemotherapy or is in remission
- Transplant patients, if the patient is on immunosuppressants or in remission
- People who use steroids, if the dose and duration of steroid use is more than two weeks, or dose is higher than 20 mg daily for prednisone
- The elderly who are bedridden, in a vegetative state, or with poor prognosis (e.g., limited life expectancy of less than 6 months)
They also say the following groups may receive the vaccine:
- Breastfeeding women
- People who have received immune globulins
They advise that for the following groups, vaccination should be deferred and rescheduled until their specific conditions are resolved:
- People with COVID-19 symptoms may be vaccinated with the COVID-19 vaccine only after full recovery
- People with history of exposure to a COVID-19 case in the past two weeks may only be vaccinated after the 14-day quarantine period
- People who have been diagnosed with COVID-19 in the past 90 days may be vaccinated after 90 days from the last day of treatment
- People who have received convalescent plasma or monoclonal antibodies for COVID-19 in the past 90 days may be vaccinated after 90 days from the last day of treatment
- People who have received any other type of vaccine in the past two weeks should be rescheduled to after the two-week interval
- Women belonging to the priority A1 group (see list of priority groups) in frontline health facilities who are in their first trimester of pregnancy may be vaccinated after the first trimester
And lastly, NITAG recommends that the following groups should not be vaccinated:
- People under 16 years of age
- People allergic to Polyethylene Glycol (PEG) and/or polysorbate
- People who experience severe allergic reactions after the first dose of the vaccine
For brand-specific guidelines, the Health Technology Assessment Council (HTAC), an advisory group to the DOH, releases guidelines for COVID-19 vaccines approved for emergency use by the FDA. As of March 10, only Pfizer-BioNTech and Oxford-AstraZeneca’s vaccines have been given these recommendations. – Sofia Guanzon/Rappler.com
This article was written by a Rappler volunteer or intern and reviewed by a member of Rappler’s research team and a senior editor. Learn more about Rappler’s internship program here.
Read Rappler’s series of explainers on the Duterte government’s vaccine program below:
- TIMELINE: The Philippines’ 2021 COVID-19 vaccine plan
- TRACKER: Which COVID-19 vaccines are being eyed by the Philippines?
- PH to prioritize high-risk areas, sectors for COVID-19 vaccine rollout
- EXPLAINER: What to expect when COVID-19 vaccines arrive in the Philippines
- EXPLAINER: How COVID-19 will get from warehouses to you
Add a comment
How does this make you feel?
There are no comments yet. Add your comment to start the conversation.