SUMMARY
This is AI generated summarization, which may have errors. For context, always refer to the full article.
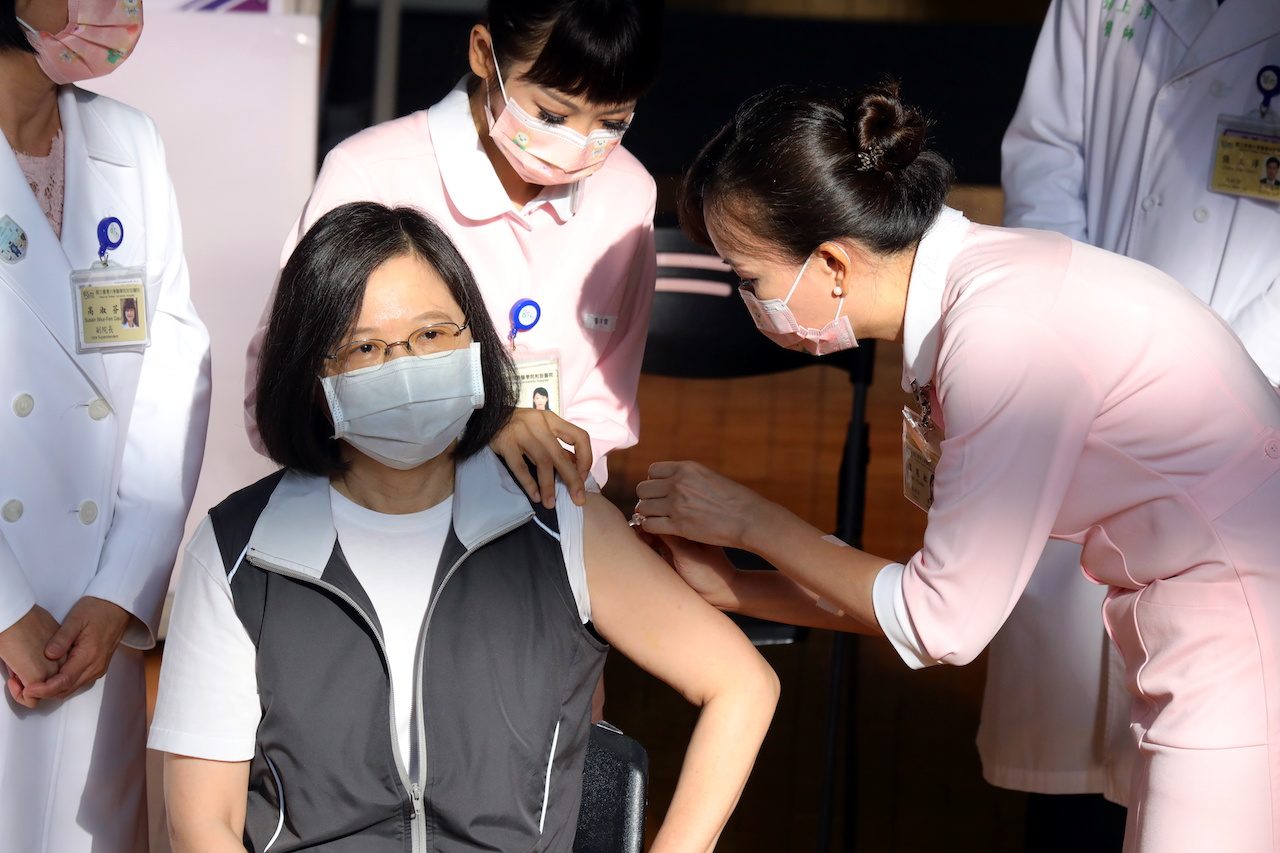
President Tsai Ing-wen got vaccinated with Taiwan’s first domestically developed COVID-19 vaccine on Monday, August 23, giving her personal stamp of approval as the island begins rolling out the contested shot whose approval critics say has been rushed.
The health ministry last month approved the emergency use of Medigen Vaccine Biologics Corp’s COVID-19 vaccine, part of a broader plan for inoculation self-sufficiency as delays in vaccine deliveries from global drug companies have affected Taiwan and many other countries.
Tsai, who had held off using vaccines from Moderna Inc or AstraZeneca Plc, the current mainstay of Taiwan’s vaccination program, received her Medigen shot at a hospital in central Taipei, demonstrating her confidence and to prove it is safe.
Tsai chatted to medical workers as they prepared her shot, the whole process being broadcast live on her Facebook page, and gave a short response of “no” to a shouted question from reporters about whether she was nervous.
More than 700,000 people have signed up so far to receive the Medigen vaccine, which requires a second shot 28 days after the first one.
Medigen, whose Chinese name literally means “high-end,” rejects claims its vaccine is either unsafe or that it has been sent to market with undue haste, saying it is effective and well-tested.
“We have done so many experiments, everyone has seen how safe our vaccine is. There are so few side effects, almost no fever and so on. So I think everyone can rest assured,” Medigen’s Chief Executive Officer Charles Chen told Reuters.
The recombinant protein vaccine has been developed in collaboration with the National Institutes of Health in the United States, and the government has ordered an initial 5 million doses. It says nobody will be forced to get it.
But Taiwan’s main opposition party, the Kuomintang, or KMT, has mounted a fierce campaign against the shot, with one of its former vice chairmen, Hau Lung-bin, filing a lawsuit to invalidate Medigen’s authorization, though a court rejected that last week.
The party says its supports domestic vaccines, but that Medigen’s approval has been rushed.
“There is no need for the lives and health of the Taiwanese people to serve as white rats in a laboratory,” Ho Chih-yung, deputy head of the KMT’s international department, told Reuters.
Around 40% of Taiwan’s 23.5 million people have received at least one shot of either of the two-dose AstraZeneca or Moderna vaccine, though fewer than 5% are fully vaccinated.
However, unlike some other parts of Asia, Taiwan faces no huge pressure to accelerate its vaccination drive, as it is recording only a handful of domestic infections a day. – Rappler.com
Add a comment
How does this make you feel?



![[Time Trowel] Evolution and the sneakiness of COVID](https://www.rappler.com/tachyon/2024/02/tl-evolution-covid.jpg?resize=257%2C257&crop=455px%2C0px%2C1080px%2C1080px)







There are no comments yet. Add your comment to start the conversation.