SUMMARY
This is AI generated summarization, which may have errors. For context, always refer to the full article.

MANILA, Philippines – The Internet brims with advice on how to conserve your smartphone battery. That’s because people aren’t satisfied yet with the solutions, both software- and hardware-based, that current consumer products offer. The processors, RAM, memory capacity – phone-makers have that covered. It’s the battery life that’s currently the bottleneck.
In a world where robots can cover a band like Nirvana, why does battery life still suck?
Majority of our rechargeable gadgets are powered by lithium-ion (Li-ion) batteries now, beating out former industry standards nickel-cadmium (Ni-Cd) and nickel metal hydride (NiMH). Li-ion uses far less hazardous materials, and is lighter while generally holding the same amount of energy as NiMH. It’s the market standard – the one that’s probably in your phone or laptop now.
It’s the type in Tesla’s electric cars too. Such is the fondness of the pioneering tech/car company for these batteries that it even built a specialized facility to make them in anticipation of future demand: the Gigafactory in Nevada, the size of which is comparable to 50 billion hamsters, according to CEO Elon Musk.
Gigafactory in units of hamster pic.twitter.com/9BAchcBX73
— Elon Musk (@elonmusk) July 30, 2016
But while Li-ion batteries have steadily increased in capacity so as to propel multi-ton objects, the progress hasn’t been fast enough to sate power-hungry mobile device users.
Here’s one promising lead: Li-ion batteries that use solid electrolytes. Electrolytes, in a nutshell, are the conductive materials encapsulated in between the positive end of a battery and the negative end. It allows the current to flow from the negative end to the positive and into your devices.
Most electrolytes today are liquid in form. What the experts are saying is that solid electrolytes as opposed to liquid electrolytes have bigger power potential. The move to solid electrolytes could be the key to dramatically increasing the juice we get out of our batteries – thus powering a more untethered existence. The hope is that we’d be able to cross out “Scan for available power sockets” from our list of things to do when visiting an establishment for the first time.
“All-solid-state batteries are the most promising candidates for future battery systems,” according to a March 2016 report in science and tech news site Phys.org. The report announced the discovery of promising new materials that may bring the world closer to high-power, fast-charging solid-state Li-ion batteries. The discovery is credited to Japanese scientists Yuki Kato and Ryoji Kanno in collaboration with colleagues from Toyota Motor Corporation, the Tokyo Institute of Technology, and High Energy Accelerator Research Organization (KEK) in Japan.
EdTech magazine shows the same enthusiasm for solid-state, which, they say, promise “longer life,” “higher performance,” and are “designed to be unbreakable.” They also note that solid-state batteries are safer because liquid electrolytes are flammable, can overheat, and cause fires.
Battery experts seem to know where they want to go. Currently, it appears to be just a matter of walking the path down and finding that magical material along the way. How long is that road exactly? Cosmin Laslau, an analyst with Lux Research – a Boston-based consultancy on emerging technologies – predicts that society won’t truly feel solid-state’s impact until 2020.
It could also be much, much sooner. In 2017, we could be seeing the “lithium-metal” battery by SolidEnergy Systems, a type that has double the power density of traditional lithium batteries.
Qichao Hu, who co-invented the new battery at the Massachusetts Institute of Technology (MIT) and is now the CEO of SolidEnergy Systems, speaks of the battery’s potential: “Industry standard is that electric vehicles need to go at least 200 miles on a single charge. We can make the battery half the size and half the weight, and it will travel the same distance, or we can make it the same size and same weight, and now it will go 400 miles on a single charge.”
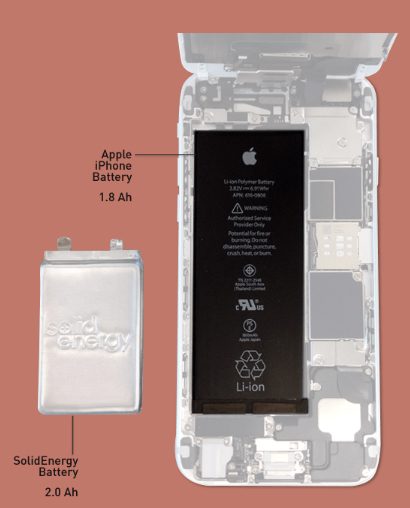
According to MIT News, Hu says the company intends to have their batteries in smartphones and wearables in 2017 and electric cars in 2018. But the first application will be drones scheduled this November. “Several customers are using drones and balloons to provide free Internet to the developing world, and to survey for disaster relief,” Hu states.
The outlook for the power bank and car charger industry just became bearish.
Other solutions on the horizon
The push for better batteries isn’t merely powered by consumers clamoring for mobile battery immortality. Tech companies themselves are participating in the race to create batteries that suit their products.
For example: Google.
The house that online search built is expanding into the industries of transportation (the Google Self-Driving Car Project), medical care (glucose-monitoring contact lenses for diabetics), and wearable devices (a successor to Google Glass?). They too want better batteries for the products they’re developing. Current Li-ion batteries with liquid electrolytes, which occasionally explode from time to time, probably aren’t the ideal solution for Google’s vision-correcting cyborg lens implant. It’s the creation of these new products – and not just the improvement of existing smartphone products – that are powering the company’s desire to develop its own batteries.

In 2009, IBM headed the Battery 500 Project that worked on a lithium-air solution, which used oxygen to stimulate the current instead of traditional liquid and solid electrolytes. The motivation was to make batteries that can power cars as reliably as gasoline. However, this foray died in 2014 as the project faced limitations on what current materials can do.
Plenty of other research and prototyping have been around the corner for quite a while. In 2016, a battery equipped with nanowire technology was introduced by the University of California, Irvine, which enables batteries to withstand over 200,000 charge cycles without degrading. Current lithium-ion setups diminish over time because some of the lithium-ions get stuck in between transport. Nanowires are so small that the lithium-ion deposits won’t build up on them.
These experiments all seem impressive even if they’re still just in the laboratories. But who knows? Down the line, one of these battery technologies – or a combination of these – might just power that ultra high-spec nano-phone implanted in your brain. – Rappler.com
Tired of your phone battery always dying on you? Why don’t you get a power bank? With power capacities ranging from 8,400mAh to 12,000mAh, choose from these coupons and discounts and get the best deals on power banks!
Add a comment
How does this make you feel?
There are no comments yet. Add your comment to start the conversation.