SUMMARY
This is AI generated summarization, which may have errors. For context, always refer to the full article.

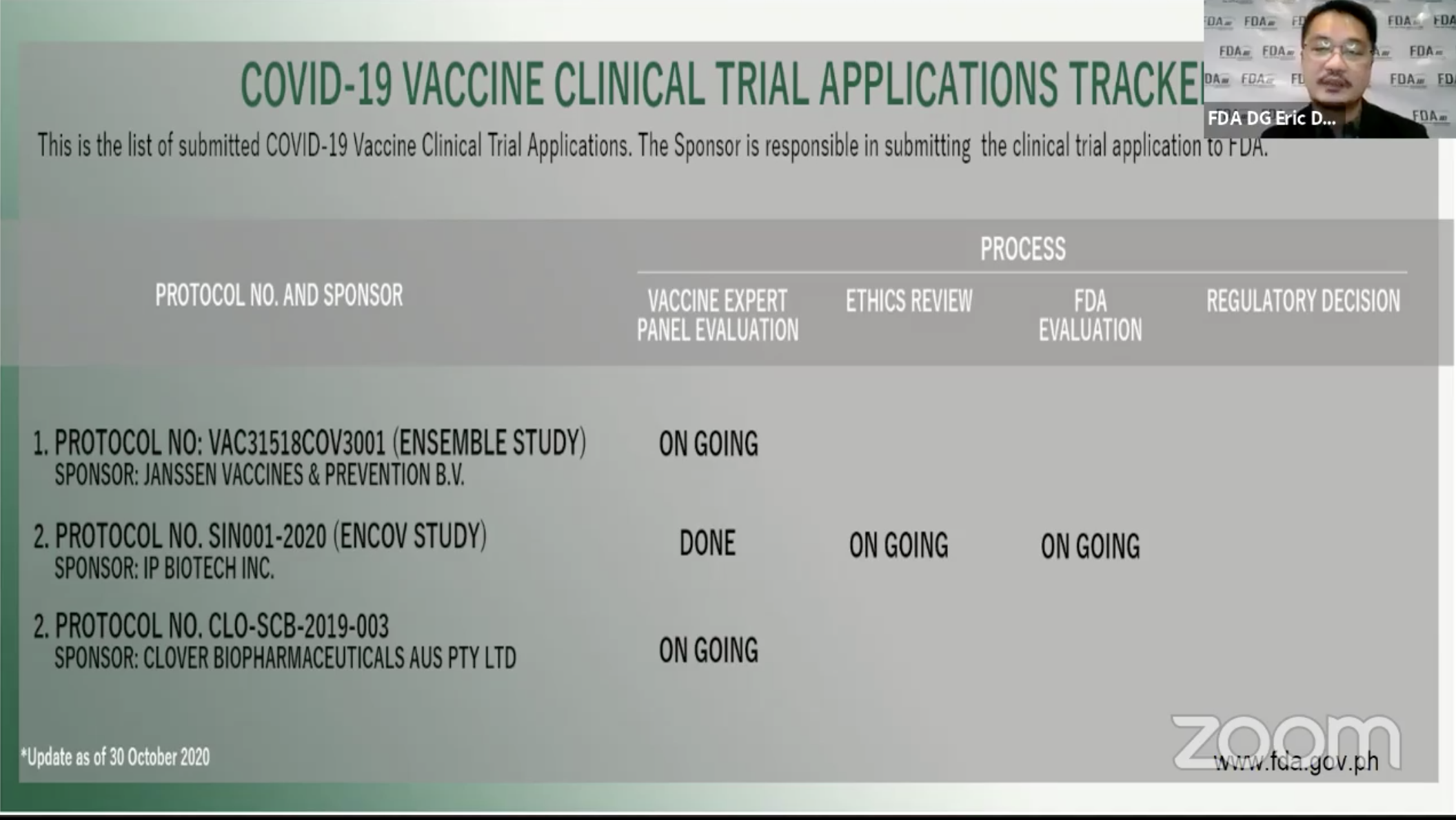
Food and Drug Administration (FDA) Director General Eric Domingo announced on Friday, October 30, that Chinese company Clover Biopharmaceuticals has applied to conduct clinical trials for the coronavirus vaccine in the country.
(Editor’s note: An earlier version of this report referred to Clover Biopharmaceuticals as an Australian company. This has been corrected.)
In a virtual press briefing on Friday morning, Domingo said that Clover has submitted its clinical trials data to the vaccine expert panel (VEP) for evaluation.
Domingo said he could not give more details about Clover as he only knew about its intent on Friday.
To date, at at least 3 companies expressed interest in conducting Phase 3 clinical trials in the Philippines. Under such trials, a vaccine is given to hundreds of people split into different age groups.
Aside from Clover, health officials cited China’s Sinovac and Russia’s Sputnik V among candidate vaccines that could possibly see independent trials in the country.
For Russia’s COVID-19 vaccine, the VEP is still evaluating data from phases 1 and 2 of its clinical trials. According to the New York Times’ vaccine tracker, this vaccine is a “combination of two adenoviruses, Ad5 and Ad26, both engineered with a coronavirus gene.”
As for Sinovac, the VEP has endorsed this to the FDA. Domingo said the agency is anticipating the submission of its application for Phase 3 trials after it gets the endorsement of the ethics board.
Clinical trials are a type of research that evaluates potential medical interventions that affect one’s health. During trials, medicines, treatments, procedures, and devices may be tested to study or verify their clinical or pharmacological effects before they are approved for public use. (READ: What we know about the Philippines’ COVID-19 vaccine plans)
In the Philippines, the FDA is the body tasked with regulating clinical trials.
Domingo said that to date, no company has been given approval to conduct clinical trials in the country.
Illegal COVID-19 vaccines
During Friday’s briefing, Domingo also warned the public against supposed coronavirus vaccines that are being sold in the market. He added that while the Philippines and other countries are doing clinical trials, these vaccines should not be promoted in the market unless they already secured FDA approval.
“We have to remember that even though may clinical trials na kahit na nagki-clinical trials na ang produkto sa Pilipinas at sa ibang bansa (there are clinical trials for products being done in the Philippines and other countries), and they are not registered, they cannot be promoted in the market as safe and effective, and definitely, they cannot be sold,” Domingo said.
This came after reports that there have been Chinese advertisements promoting COVID-19 vaccines priced at P50,000.
“Ang puwede lang magamit ng madla ay ‘yung mga rehistrado lang ng FDA (The public can only use those that are FDA-registered),” Domingo stressed. – Rappler.com
Add a comment
How does this make you feel?
There are no comments yet. Add your comment to start the conversation.