SUMMARY
This is AI generated summarization, which may have errors. For context, always refer to the full article.

MANILA, Philippines – The medical community has been divided by the stem cell therapy issue, with practitioners disagreeing on whether treatments should be allowed without clinical trials being approved by the Food and Drug Administration (FDA).
For Health Secretary Enrique Ona, to encourage medical innovations, local clinical trials or thorough drug testing prior to a drug’s release to the market can be foregone in the meantime for stem cell and stem cell-based products.
In his speech during the mid-year convention of the Philippine Society for Stem Cell Medicine (PSSCM), Ona acknowledged that “predetermined adherence to a protocol might be a fatal rigidity.”
But 21 medical and surgical societies found the health secretary’s stand problematic, prompting them to issue a position statement calling for stricter regulation. The National Insitute of Health of the University of the Philippines Manila supported the dissenting doctors.
Dr Antonio Dans, an epidemiologist and the president of the Philippine Society of General Internal Medicine, believes it is unethical to charge patients for treatments that are not proven and have not undergone clinical trials.
Stem cell therapy is a medical intervention that involves extracting the body’s repair cells and injecting them back to the body to replace old cells. Animal-sourced or xeniobiotic stem cells are restricted by the Department of Health (DOH). (READ: 6 things you need to know about stem cell therapy)
The challenge now hounding the DOH and the FDA is “finding the balance between innovation and sound regulation.”
Long and arduous clinical trials
Must we evade traditional protocol to give way to innovation? Clinical trials are long and arduous. They are meant to determine a drug’s proven indications, a clearly indicated therapeutic purpose to inform the patient of the drug’s benefits and risks.
Liberal regulators and stem cell transplant practitioners argue that the protocol will cripple the development of the treatment, especially since it will mean that pending clinical trial results, the therapy will have to be banned from the market.
“It is going to take years or even decades before we can have a list of ‘proven indications,'” said stem cell transplant physician Dr Florencio Lucero, who started doing the procedure 6 years ago. (READ: DOH: Stem cell therapy not yet proven to be curative)
As of August 1, no application with the FDA for a clinical trial involving a stem cell or stem cell-based product has been made. The DOH’s current guidelines, however, already lists prohibited and restricted types of stem cell treatment.

Unproven indications
Ona said offering free treatments might relegate the country to being a mere testing ground for the treatment.
He added that vascular and organ transplant procedures used to be unaccepted treatments in the 60s but were eventually considered life-saving by the medical community.
Detractors rejected Ona’s parallelism, saying that organs are not products subject to FDA regulation. But some will argue neither are cells.
The concoction injected, however, during a stem cell treatment can be reviewed by the FDA once clinical trial results are submitted.
Clinical trials
Dans stressed the need for clinical trials, as they are designed to determine the efficacy and safety of a drug.
Dr Samuel Bernal, stem cell transplant practitioner from The Medical City, said clinical trials are too costly and could stifle the development of the treatment.
“I am very much for evidence, but there are many ways to collect evidence,” he said, explaining that research initiatives and partnerships with academic institutions are in place anyway.
Lucero said he has conducted clinical trials for stem cell therapy on 30 diabetic patients and have seen good results thus far. The trials however did not use control groups, and results will not be accepted by other physicians or published in peer-reviewed journals.
“It is something that [is] going against the grain. Unfortunately, all of us doctors in the Philippines are trained the Western medicine way. Meaning, we rely on evidence-based medicine. Any clinical trial you do that is going against the grain is not acceptable to majority (or) practically all physicians,” he said in a phone interview.

Need for control groups
Dans, however, explained the need for control groups to reduce external factors that may lead to cure or improved conditions.
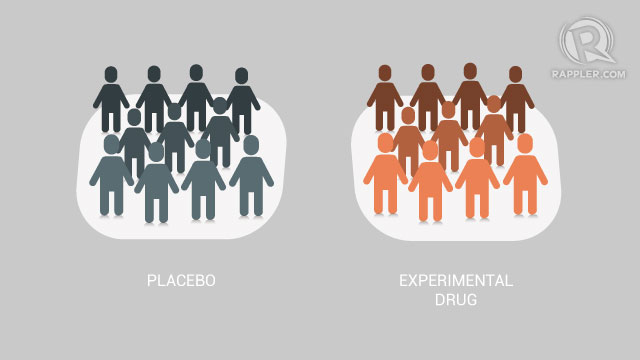
He explained that physical improvements may simply be a placebo effect, be the result of concurrent treatments received, or be caused by the body’s natural capacity to heal itself.
Two randomized control groups — one group administered with either the placebo drug or the current treatment, and the other group administered with the new treatment being tried — are the minimum requirement.
Comparing the two groups allows clinicians to see the difference between the drug’s effect versus other possible causes of cure.
The difference between the effect of the placebo and the effect of the new treatment demonstrates how effective the drug is.
Differences in body make-up
To Bernal, the difference in body make-up at the molecular level makes the process “very complicated.” This complication extends to the design of clinical trials.
He said it would be hard to randomize groups and compare them, given that no two patients are the same.
“It is exactly because each patient is different and exactly because only some get cured that we need randomized clinical trials,” Dans countered.

Drugs with special permits
To be sure, not all drugs administered to patients here have undergone clinical trials in the country. There are medicines provided with special permits that allow administration to a patient.
Once the necessary permit is secured, the unregistered drug can be imported and used.
Dr Tito King, head of the FDA division that grants these special permits, said the basic rationale is that the “welfare of the patient should not be held hostage by registration.”
These special cases that allow the importation of locally unregistered drugs include:
- compassionate use (when the patient is terminally ill, has no other medical alternative, and consents to an experimental drug);
- a patient’s change of location (when a patient abroad was prescribed a drug not registered locally, and he/she migrates to, or vacations in, the Philippines); and
- essential use (when the drug is not registered locally but is the life-saving drug available for the disease being treated)
Drugs administered by hospitals for essential use are usually for rare diseases, where the local market is too small that no pharmaceutical company has invested in its sale in the country.
But do stem cell products fall within these categories?
Last resort
Stem cell products are special, given their potential. But they are not part of the “standard of care,” that is generally accepted as a mode of treatment.
Often, they must be administered as a last resort when all other treatments have failed.
“Patients who come for this procedure are desperate for improvement, because of lack of significant improvement from the [standard] medication or treatment for many years,” Lucero said.
The criterion that may permit experimental stem cell therapies is “compassionate use” meant for terminally ill patients with a life expectancy of 6 months or less.
There are, however, stem cell transplant practitioners who offer stem cell therapy for conditions other than terminal illnesses such as diabetes, stroke, or even simply aging. (READ: ‘Suspend deceptive stem cell transplant doctors’)
Give patients a chance
Members of the PSSCM say the treatment offers hope that cannot be ignored.
“I inform them of the possibility. No promise of cure,” said Lucero, adding that there is nothing wrong with administering the therapy so long as the patient agrees to it after being informed about its limitations.
Another stem cell transplant physician, Dr Cristina Puyat, said the curative effects differ but there’s qualitative improvement for sure.
“Some patients really get cured, [while] some don’t. What definitely happens is that there’s improvement in the quality of life. So, I think, patients should also be given the chance,” she said.
To dissenters, however, this “chance” comes at the expense of ethics and protocol supposedly valued by the medical profession. – Rappler.com
Add a comment
How does this make you feel?
There are no comments yet. Add your comment to start the conversation.