SUMMARY
This is AI generated summarization, which may have errors. For context, always refer to the full article.
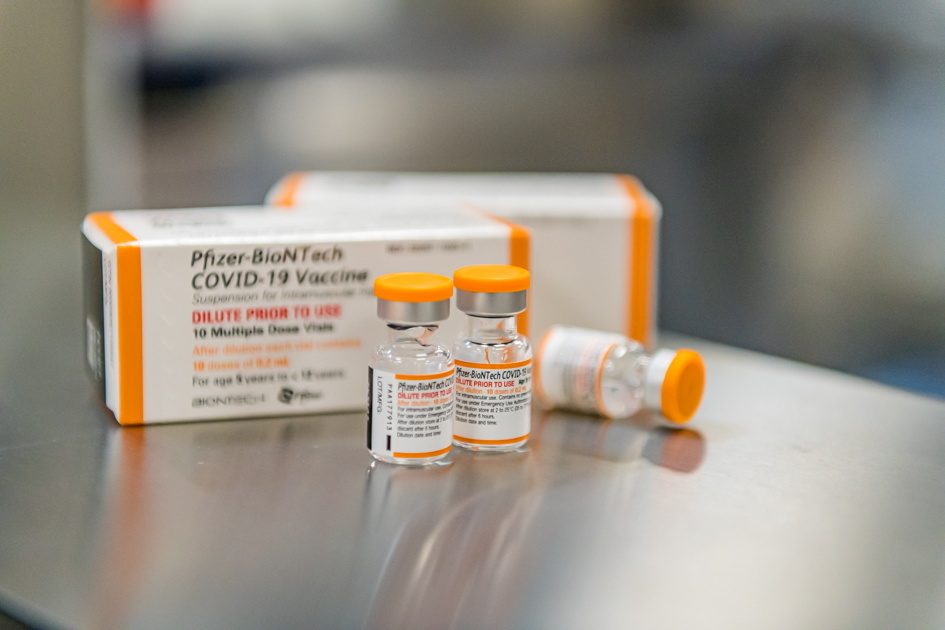
Pfizer said on Monday, November 22, its COVID-19 vaccine provided strong long-term protection against the virus in a late-stage study conducted among adolescents aged 12 to 15 years.
A two-dose series of the vaccine was 100% effective against COVID-19, measured seven days through over four months after the second dose, the company said.
The long-term data will support planned submissions for full-regulatory approval of the vaccine in the age group in the United States and worldwide.
Pfizer and BioNTech will seek clearance for a 30-microgram dose of the vaccine for those aged 12 and above.
The vaccine was authorized for emergency use in people aged 12-15 years by the U.S. Food & Drug Administration in May, and granted full approval for use in people aged 16 and above in August. – Rappler.com
Add a comment
How does this make you feel?


![[Time Trowel] Evolution and the sneakiness of COVID](https://www.rappler.com/tachyon/2024/02/tl-evolution-covid.jpg?resize=257%2C257&crop=455px%2C0px%2C1080px%2C1080px)



There are no comments yet. Add your comment to start the conversation.