SUMMARY
This is AI generated summarization, which may have errors. For context, always refer to the full article.
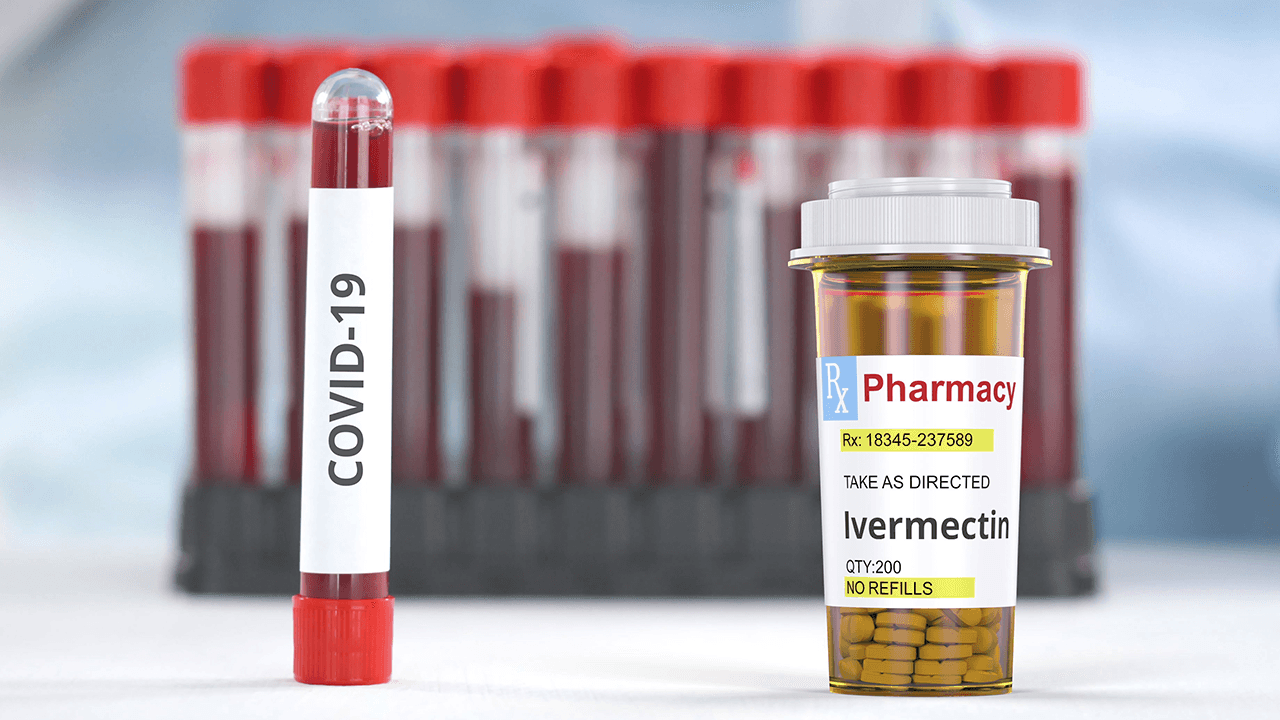
MANILA, Philippines – The United States Food and Drug Administration (FDA) has settled a lawsuit brought by Texan doctors about the agency’s statements against using ivermectin as a treatment drug for COVID-19.
Physicians Mary Talley Bowden, Robert Apter, and Paul Marik argued that the FDA went beyond its jurisdiction. Marik said the agency “interfered with the practice of medicine with their irresponsible language and posts about ivermectin.”
“We will never know how many lives were affected because patients were denied access to a lifesaving treatment because their doctor was just following the FDA,” said Marik, who is also chairman and chief scientific officer at the Front Line COVID-19 Critical Care Alliance (FLCCC Alliance).
As a result of the settlement, which was dated March 21, the FDA will be deleting social media posts related to the drug, specifically posts with the caption: “You are not a horse. Stop it with the #ivermectin. It’s not authorized for treating COVID,” among others.
The agency was also ordered to take down within 21 days its web page titled “Why You Should Not Use Ivermectin to Treat or Prevent COVID-19.”
According to Newsweek, the FDA said it moved to settle “rather than continuing to litigate over statements that are between two and nearly four years old.”
In a statement sent to The Epoch Times, the FDA maintained that clinical trial data does not show the drug’s effectiveness against COVID-19.
For animals, for humans
There are two kinds of ivermectin. One is for animals, which is used to prevent parasites in cows and horses. Meanwhile, the one approved for human use has a different concentration and is prescribed for those with head lice and other conditions such as rosacea (“a common skin condition that causes flushing or long-term redness on your face,” according to Mayo Clinic).

Not banned in the Philippines
Back home, in 2021, the Philippines’ own FDA also issued a warning against using ivermectin for COVID-19.
There was a rise in online posts about the drug as a viable treatment at the height of the pandemic, but online listings of ivermectin in the country only sold the animal variant.
While ivermectin is not banned in the Philippines, it is registered as a prescribed topical medicine. This means that people could only get access to the proper ivermectin variant if they had a doctor’s prescription.
“Any use of ivermectin veterinary products for the prevention or treatment of COVID-19 should be avoided as the benefits and safety for this purpose has not been established,” Manila’s FDA said in a March 15, 2021 advisory.
In April 2021, the FDA allowed a hospital to use ivermectin to treat a COVID-19 patient through a compassionate special permit. The number had grown to six hospitals, as of May 2021.
US FDA engaged in ‘fear-mongering’
Physicians Bowden, Apter, and Marik celebrated the agency’s settlement, noting that the FDA’s statements regarding ivermectin were essentially a form of fear-mongering.
The FLCCC Alliance also said that the statements “illegally interfered with the doctors’ ability to practice medicine.”
“This landmark case sets an important precedent in limiting FDA overreach into the doctor-patient relationship,” Bowden said.
🚨BREAKING:
— Mary Talley Bowden MD (@MdBreathe) March 22, 2024
FDA loses its war on ivermectin and agrees to remove all social media posts and consumer directives regarding ivermectin and COVID, including its most popular tweet in FDA history.
This landmark case sets an important precedent in limiting FDA overreach into the… pic.twitter.com/HWYkkZLpoJ
The case was first filed with the US District Court in June 2022, but was dismissed after the court found the FDA to have “sovereign immunity,” the FLCCC Alliance recalled. The US Court of Appeals for the Fifth Circuit reversed the case’s dismissal in September 2023.
“[The] FDA can inform, but it has identified no authority allowing it to recommend consumers ‘stop’ taking medicine,” the US Court of Appeals said. – Rappler.com
Add a comment
How does this make you feel?

![[Rappler’s Best] US does propaganda? Of course.](https://www.rappler.com/tachyon/2024/06/US-does-propaganda-Of-course-june-17-2024.jpg?resize=257%2C257&crop=236px%2C0px%2C720px%2C720px)




![[ANALYSIS] Hippocrates and hypocrites](https://www.rappler.com/tachyon/2024/07/TL-medical-ethics-june-27-2024.jpg?resize=257%2C257&crop=314px%2C0px%2C720px%2C720px)
![[Free to disagree] Why investigate Bell-Kenz Pharma Inc?](https://www.rappler.com/tachyon/2024/05/TL-why-investigate-punish-bell-kenz-pharma-inc-May-6-2024.jpg?resize=257%2C257&crop_strategy=attention)



![[OPINION] On Ivermectin, COVID-19 response, and the business sector](https://www.rappler.com/tachyon/2021/05/tl-sq-1.jpg?resize=257%2C257&crop_strategy=attention)





There are no comments yet. Add your comment to start the conversation.