SUMMARY
This is AI generated summarization, which may have errors. For context, always refer to the full article.
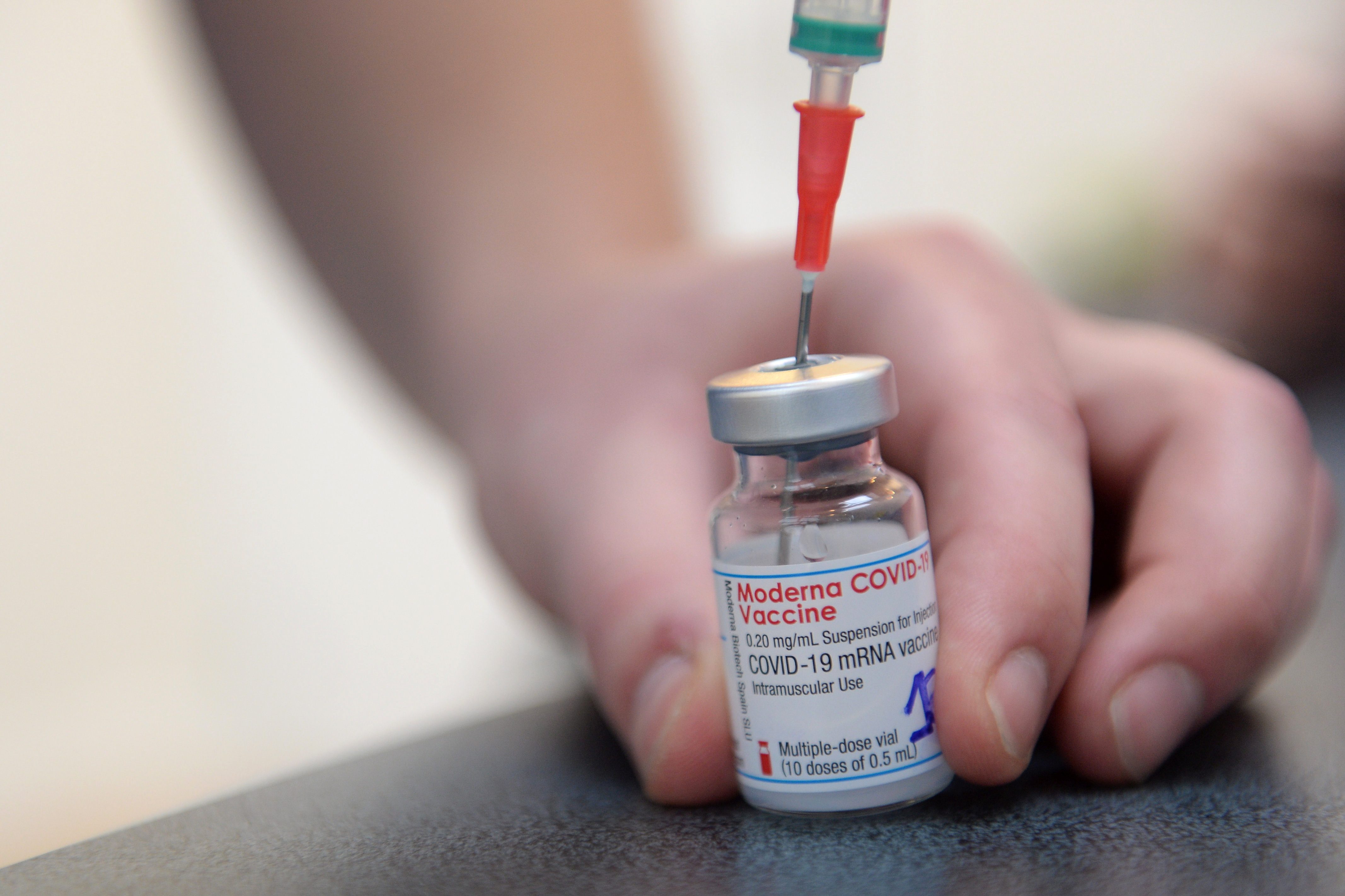
American drug firm Moderna has filed an application for emergency use authorization of its coronavirus vaccine in the Philippines, the Food and Drug Administration (FDA) said on Monday, April 26.
FDA Director General Eric Domingo confirmed this in a message to Rappler, saying the application was filed on Monday afternoon.
Moderna is the eighth vaccine company to apply for emergency use of its vaccine in the country. If approved, it would be the seventh to become available for public use. The filing with the FDA is a significant step in allowing Filipinos access to its vaccine within the year, with initial doses expected to arrive sometime from May to June.
The FDA is expected to take at least 21 days to review the vaccine’s trial data and other information to determine the quality, safety, and efficacy of the shot on the Philippine population.
Like other vaccines approved by stringent regulatory authorities, Moderna’s review could move swiftly with at least 46 other countries granting it similar emergency use authority as of April 19. Moderna’s vaccine has been one of three shots used in the United States’ vaccination program, along with Pfizer and Johnson and Johnson’s (Janssen Pharmaceutical) vaccines.
Why this matters
Clearing Moderna’s COVID-19 vaccine will provide another option for the Philippines, which is battling one of the worst outbreaks in Southeast Asia. The country’s vaccine campaign, launched on March 1, is also badly in need of more vaccines as supplies remain severely limited and its schedule of deliveries had been setback by delays due to erratic global supply.
The Philippines earlier ordered 20 million doses of Moderna’s vaccine for 2021 and is eyeing to purchase millions of booster shots being developed by the drug firm to provide additional immunity against COVID-19.
In terms of performance, preliminary data published in the New England Journal of Medicine found that Moderna’s vaccine is more than 90% effective against COVID-19 about six months after the second dose of the shot is administered. Phase 3 trial data showed the vaccine is also 94% effective against symptomatic COVID-19 14 days after the second dose.
Storage of Moderna’s vaccine would be more complex compared to other jabs as it requires being frozen in colder temperatures, specifically -25°C to -15°C up to expiration. Unlike Pfizer’s vaccine, however, Moderna’s shot may be kept longer in standard refrigerated temperates of 2°C to 8°C for 30 days, making it easier to handle for countries like the Philippines equipped with such storage facilities.
Moderna earlier said it was studying immune responses to its vaccine beyond six months and whether its booster shot would be effective against variants of COVID-19.
The Philippines earlier cleared the vaccines of Pfizer and BioNTech, AstraZeneca, Sinovac, Gamaleya Research Institute, J&J, and Bharat Biotech for emergency use in the country. – Rappler.com
Add a comment
How does this make you feel?



![[Time Trowel] Evolution and the sneakiness of COVID](https://www.rappler.com/tachyon/2024/02/tl-evolution-covid.jpg?resize=257%2C257&crop=455px%2C0px%2C1080px%2C1080px)




![[PANOORIN] Naku! Mag-ingat sa unregistered health products online!](https://www.rappler.com/tachyon/2023/05/fact-check-ls-3.jpg?resize=257%2C257&crop=283px%2C0px%2C720px%2C720px)

There are no comments yet. Add your comment to start the conversation.